Tipe Molekul dan Geometri Molekul
| Jumlah Ikatan | Pasangan Elektron Terikat | Pasangan Elektron Bebas | Tipe Molekul | Geometri Molekul |
| 2 | 2 | 0 | \(\ce{AX2}\) |
Linear |
| 3 | 3 | 0 | \(\ce{AX3}\) |
Segitiga datar |
| 4 | 4 | 0 | \(\ce{AX4}\) |
Tetrahedral |
| 5 | 5 | 0 | \(\ce{AX5}\) |
Bipiramida segitiga |
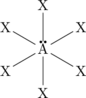
| 6 | 6 | 0 | \(\ce{AX6}\) |
Oktahedral |
| 4 | 3 | 1 | \(\ce{AX3E}\) |
Oktahedral |
| 4 | 2 | 2 | \(\ce{AX2E2}\) |
Bentuk V |
| 5 | 4 | 1 | \(\ce{AX4E}\) |
Jungkat-jungkit (see-saw) |
| 5 | 3 | 2 | \(\ce{AX3E2}\) |
Bentuk T |
| 5 | 2 | 3 | \(\ce{AX2E3}\) |
Linear |
| 6 | 4 | 2 | \(\ce{AX4E2}\) |
Segiempat datar
|
| 7 | 6 | 1 | \(\ce{AX6E}\) |
Tetrahedral terdistorsi (menyimpang) |
SOAL LATIHAN

![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}A(-[:90]@{2}X)(-[:210]@{3}X)(-[:330]@{4}X)} \arclabel{0.5cm}{3}{1}{4}{\footnotesize \SI{120}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-bdcc12d5204b4c66186e1322056ee06c_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}A(-[:90]@{2}X)(-[:199.5]@{3}X)(<[:300]@{4}X)(<:[:350]@{5}X)} \arclabel{0.5cm}{3}{1}{2}{\footnotesize \SI{109.5}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-37bf00b46dca594462f97fb0f3ba60b0_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}A(-[:0]@{2}X)(-[:150]@{3}X)(-[:210]@{4}X)(-[:90]@{5}X)(-[:270]@{6}X)} \arclabel{0.5cm}{3}{1}{4}{\footnotesize \SI{120}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-bd3aae60f93d9443852bb8e711356419_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}A(-[:30]@{2}X)(-[:150]@{3}X)(-[:210]@{4}X)(-[:330]@{5}X)(-[:90]@{6}X)(-[:270]@{7}X)} \arclabel{0.5cm}{3}{1}{4}{\footnotesize \SI{90}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-20fb9e2724b81bfb3ec6ceed30864f3a_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}\charge{90=\:} {A}(-[:199.5]@{3}X)(<[:300]@{4}X)(<:[:350]@{5}X)} \arclabel{0.5cm}{3}{1}{4}{\footnotesize \SI{100}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-24853b8ad1cbf1186d6d598f52640c5b_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1}\charge{0=\:} {A}(-[:150]@{3}X)(-[:210]@{4}X)(-[:90]@{5}X)(-[:270]@{6}X)} \arclabel{0.5cm}{3}{1}{4}{\footnotesize \SI{120}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-210d1399a16884fee3b7b41719cec503_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1} \charge{135=\:,225=\:} {A}(-[2]@{2}X)(-[6]@{3}X)-@{4}X} \arclabel{0.5cm}{2}{1}{4}{\footnotesize \SI{90}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-5bbd0c6155d33324e36c1026aef8cd6e_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1} \charge{0=\:,135=\:,225=\:} {A}(-[2]@{2}X)(-[6]@{3}X)} \arclabel{0.5cm}{2}{1}{3}{\footnotesize \SI{180}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-5f886436079753174592d2df1760ea60_l3.png)
![Rendered by QuickLaTeX.com \begin{document} \chemfig{@{1} \charge{90=\:} {A}(-[:30]@{2}X)(-[:150]@{3}X)(-[:210]@{5}X)(-[:330]@{7}X)} \arclabel{0.5cm}{2}{1}{3}{\footnotesize \SI{90}{\degree}} \end{document}](https://clavius.id/wp-content/ql-cache/quicklatex.com-63b79a9ea313266cc0de9229dd5d2570_l3.png)