Persamaan Laju Reaksi
Persamaan laju reaksi didapat dari data hasil percobaan.
\(\ce{A(g) + B(g) -> C(g)}\)
Persamaan laju reaksi:
\(v = k \:.\: [A]^x \:.\: [B]^y \)
\(x\) dan \(y\) adalah orde reaksi masing-masing zat.
Orde reaksi total = \(x + y\)
Grafik Laju Reaksi vs Konsentrasi
\(v = k \: [A]^0\)

\(v = k \: [A]^1\)

\(v = k \: [A]^2\)

\(v = k \: [A]^{-1}\)
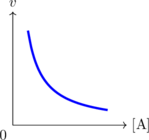
\(v = k \: [A]^{\frac 12}\)

SOAL LATIHAN
