Oksida Asam
\(\ce{UNSUR NONLOGAM + O2 -> OKSIDA ASAM}\)
A. Pembentukan Oksida Asam
Contoh:
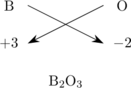
Boron dengan bilangan oksidasi +3 dan oksigen dengan bilangan oksidasi −2 dapat membentuk oksida asam \(\ce{B2O3}\)
B. Tatanama Oksida Asam
-
-
- Oksida asam terdiri atas unsur nonlogam dan oksigen. Penamaan oksida asam terdiri atas "nama unsur" ditambahkan dengan kata "oksida"
-
-
-
- Penamaan oksida asam menggunakan awalan:
-
mono = 1
di = 2
tri = 3
tetra = 4
penta = 5
heksa = 6
hepta = 7
okta = 8
nona = 9
Kecuali jika jumlah atom unsur nonlogam = 1, tidak boleh ditambahkan mono
Contoh:
\(\ce{N2O3}\) = dinitrogen trioksida
\(\ce{P2O5}\) = difosfor pentaoksida
\(\ce{CO2}\) = karbon dioksida (bukan monokarbon dioksida)
\(\ce{CO}\) = karbon monooksida (bukan monokarbon monooksida)
C. Contoh-contoh
| Unsur | + | \(\ce{O2}\) | \(\ce{->}\) | Oksida Asam |
|---|---|---|---|---|
| B
Bilangan oksidasi: +3 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{B2O3}\)
Diboron trioksida |
| C
Bilangan oksidasi: +4 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{CO2}\)
Karbon dioksida |
| Si
Bilangan oksidasi: +4 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{SiO2}\)
Silikon dioksida |
| S
Bilangan oksidasi: +4 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{SO2}\)
Belerang dioksida |
| S
Bilangan oksidasi: +6 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{SO3}\)
Belerang trioksida |
| N
Bilangan oksidasi: +3 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{N2O3}\)
Dinitrogen trioksida |
| N
Bilangan oksidasi: +5 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{N2O5}\)
Dinitrogen pentaoksida |
| P
Bilangan oksidasi: +3 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{P2O3}\)
Difosfor trioksida |
| P
Bilangan oksidasi: +5 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{P2O5}\)
Difosfor pentaoksida |
| As
Bilangan oksidasi: +3 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{As2O3}\)
Diarsen trioksida |
| As
Bilangan oksidasi: +5 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{As2O5}\)
Diarsen pentaoksida |
| Sb
Bilangan oksidasi: +3 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{Sb2O3}\)
Diantimon trioksida |
| Sb
Bilangan oksidasi: +5 |
+ | \(\ce{O2}\)
Bilangan oksidasi: −2 |
\(\ce{->}\) | \(\ce{Sb2O5}\)
Diantimon pentaoksida |
Asam
\(\ce{OKSIDA ASAM + H2O -> ASAM}\)
| Oksida Asam | + | \(\ce{H2O}\) | \(\ce{->}\) | Asam |
|---|---|---|---|---|
| \(\ce{CO2}\)
Karbon dioksida |
+ | \(\ce{H2O}\) | \(\ce{->}\) | \(\ce{H2CO3}\)
Asam karbonat Asam karbonat (IV) |
| \(\ce{SO2}\)
Belerang dioksida |
+ | \(\ce{H2O}\) | \(\ce{->}\) | \(\ce{H2SO3}\)
Asam sulfit Asam sulfat (IV) |
| \(\ce{SO3}\)
Belerang dioksida |
+ | \(\ce{H2O}\) | \(\ce{->}\) | \(\ce{H2SO4}\)
Asam sulfat Asam sulfat (VI) |
| \(\ce{N2O3}\)
Dinitrogen trioksida |
+ | \(\ce{H2O}\) | \(\ce{->}\) | \(\ce{2 HNO2}\)
Asam nitrit Asam nitrat (III) |
| \(\ce{N2O5}\)
Dinitrogen pentaoksida |
+ | \(\ce{H2O}\) | \(\ce{->}\) | \(\ce{2 HNO3}\)
Asam nitrat Asam nitrat (V) |
| \(\ce{P2O3}\)
Difosfor trioksida |
+ | \(\ce{3 H2O}\) | \(\ce{->}\) | \(\ce{2 H3PO3}\)
Asam fosfit Asam fosfat (III) |
| \(\ce{P2O5}\)
Difosfor pentaoksida |
+ | \(\ce{3 H2O}\) | \(\ce{->}\) | \(\ce{2 H3PO4}\)
Asam fosfat (V) |
| \(\ce{As2O3}\)
Diarsen trioksida |
+ | \(\ce{3 H2O}\) | \(\ce{->}\) | \(\ce{2 H3AsO3}\)
Asam arsenit Asam arsenat (III) |
| \(\ce{As2O5}\)
Diarsen pentaoksida |
+ | \(\ce{3 H2O}\) | \(\ce{->}\) | \(\ce{2 H3AsO4}\)
Asam arsenat Asam arsenat (V) |
Catatan:
Khusus oksida boron (B), fosfor (P), arsen (As) dan antimon (Sb), ditambahkan 3 molekul \(\ce{H2O}\)
Pembentukan asam lainnya
| Sulfur dan Halogen | + | \(\ce{H2}\) | \(\ce{->}\) | Asam |
|---|---|---|---|---|
| \(\ce{S}\)
Sulfur |
+ | \(\ce{H2}\) | \(\ce{->}\) | \(\ce{H2S}\)
Asam sulfida |
| \(\ce{F2}\)
Belerang dioksida |
+ | \(\ce{H2}\) | \(\ce{->}\) | \(\ce{2 HF}\)
Asam fluorida |
| \(\ce{Cl2}\)
Belerang dioksida |
+ | \(\ce{H2}\) | \(\ce{->}\) | \(\ce{2 HCl}\)
Asam klorida |
| \(\ce{Br2}\)
Dinitrogen trioksida |
+ | \(\ce{H2}\) | \(\ce{->}\) | \(\ce{2 HBr}\)
Asam bromida |
| \(\ce{I2}\)
Dinitrogen pentaoksida |
+ | \(\ce{H2}\) | \(\ce{->}\) | \(\ce{2 HI}\)
Asam iodida |
Asam Karboksilat
| Rumus Kimia | Nama Senyawa |
|---|---|
| \(\ce{CHOOH}\) | Asam formiat (asam semut) |
| \(\ce{CH3COOH}\) | Asam asetat (asam cuka) |
SOAL LATIHAN
